| Wednesday, 21 May 2014 | |
|---|---|
| 8:30 am | Registration |
| 9:00 am – 6:30 pm | Workshop (including Poster Session) |
| Thursday, 22 May 2014 | |
|---|---|
| 8:30 am | Registration |
| 9:00 am – 3:25 pm | Workshop (including Prize-giving ceremony) |
| 3:30 pm | Visit of Château de Versailles |
Download a pdf of the detail conference programme.
Click on speaker names below to see an abstract of their respective talks.
Conference Speakers
Day-1, May 21, 2014
Cardiac Electrophysiology
Session chair: Anne Dubin, MD: Stanford, USA
-
Scott Ceresnak, MD: Stanford University, USA
Scott Ceresnak, MD: Stanford University, USA
Device Therapy In Children and Patients with Congenital Heart Disease
Though the equipment used for a pacemaker or defibrillator implant is identical in children and adults, there are a plethora of unique issues pertinent to the pediatric and congenital heart disease populations. Small body habitus, venous access issues, long-term planning for lifelong pacing, anatomical variation, and issues related to surgical palliation and repair all factor into device decisions in these patients. In this lecture we will review the basics of pacemakers and implantable-cardioverter defibrillators (ICDS), the indications for pacing in children and patients with congenital heart disease, the unique aspects of pacing in these populations, and discuss the future of device therapy.
-
Yves Coudiere, PhD: INRIA, France
Yves Coudiere, PhD: INRIA, France
Cardiac electrophysiology modeling from the ion channel to the organ and body levels: two examples from pharmacological and clinical studies.
Cardiac electrophysiology modeling has evolved incredibly over the past 50 years, providing highly detailed mathematical description from the molecular to the organ level. Cardiac electrical activity is determined by the molecular activity through the cell membrane that is organized at the tissue, organ and body levels. Cardiac arrhythmias are complex disruptions of this organization. Numerical models and fast dedicated solvers now allow in-silico exploration of the mechanisms underlying these pathologies. Therefore, close collaborations between clinicians, electrophysiologists and modelers are required. I will describe two results from successful collaborations of this kind. First I will explain how our softwares can provide insight into the effects of drugs from the ion channel level to the electrocardiogram. Then I will show how mathematical modeling and numerical simulation was used to explore the mechanisms of a cardiac channelopathy. The objective of this second study was to describe a familial cardiac phenotype and elucidate the electrophysiological mechanisms responsible for the disease. Mutations in the SCN5A gene were identified in several individuals within unrelated families, that were affected with multifocal ectopic premature contractions. The mutation was characterized by patch-clamp experiments and studied in vitro and in silico with the numerical models. The numerical experiments gave a innovative insight into the mechanisms of the pathology, that confirmed the role of the Purkinje system in the pathology, and also the success of the hydroquinidine treatment for these patients. Although for different reasons, we will see that high-performance computing was required for both in-silico studies. These studies enlighten the necessity and scientific interest of collaborations between computer science, mathematical and biophysical modeling together with clinicians and experimentalists.
-
Weiguang Yang, PhD: Stanford University, USA
Weiguang Yang, PhD: Stanford University, USA
Simulating Venous Flow with Pacemaker/ICD leads in Pediatric Patients
Sarah E. Stewart, Anne M. Dubin, Jeffrey A. Feinstein, Weiguang Yang
Background: Pediatric transvenous pacemakers and implantable cardioverter defibrillators (ICDs) have an increased risk of venous occlusion when compared to the adult population. This increased risk has been attributed to smaller blood vessels and relatively larger leads. This study aimed to characterize how patient (vessel) and lead sizes influence the likelihood and location of venous occlusions in pediatric patients.
Methods: Image-based computational fluid dynamics (CFD) was used for blood flow simulation. Patient specific models were constructed from CT images for three pediatric patients (age 12-15 years). To quantify flow stagnation, a Lagrangian particle-tracking code was employed to calculate the mean exposure time (MET), the time particles reside in each tetrahedral element. Pulsatile inflow and resistance boundary conditions were applied to the model inlets and outlet respectively. Three vessel sizes representing ages 5-8, 8-10 and 10-12 years were then modeled with and without leads to assess venous stasis. Three lead sizes (5, 7.8 and 11 Fr) were compared in a patient with a single-lead pacemaker.
Results: Simulations showed smaller patients to have more stasis. Mean wall shear stress (WSS) increased with smaller vessel size. Highest MET was found between the leads and vessel wall in all patient groups. Decreases in velocity and increases in MET (which may indicate venous occlusion) are greatest in the smallest age group, and located between the two leads, and between the leads and vessel walls (figure). MET decayed rapidly with distance away from the leads and vessel walls. WSS increased with increasing lead size.
Conclusion: CFD is capable of evaluating the impacts of vessel and lead sizes on blood flow. Decreased effective cross sections for blood flow resulted in elevated MET. Future work may assist in optimal lead design, placement, and patient risk stratification.
-
Olivier Bernus, PhD : University Bordeaux Segalen, France
Olivier Bernus, PhD : University Bordeaux Segalen, France
Pro-arrhythmic ventricular remodeling in a porcine model of repaired tetralogy of Fallot
DUBES Virginie1,2, BENOIST David1,2, ROUBERTIE François1,2,3, GILBERT Steve1,2, CONSTANTIN Marion1,2, ELBES Delphine1,2, VIELLOT Delphine4, CHARRON Sabine1,2, COCHET Hubert1,2,3, QUESSON Bruno1,2, ROORYCK-THAMBO Caroline1,3, BORDACHAR Pierre1,2,3, HAISSAGUERRE Michel1,2,3, THAMBO Jean- Benoit1,2,3, BERNUS Olivier1,2
1 L’Institut de Rythmologie et Modélisation Cardiaque, Université Bordeaux, Pessac 2 Inserm U1045 CRCTB, Université Bordeaux Segalen, Bordeaux 3 CHU de Bordeaux, Bordeaux 4 Plateforme Technologique d’Innovation Biomédicale, PessacVentricular arrhythmias are frequent in patients with repaired tetralogy of Fallot but their underlying mechanisms remain unclear. In this study, ventricular electrical and structural remodelling was assessed in an animal model that mimics postoperative tetralogy of Fallot.
Piglets underwent a tetralogy of Fallot repair-like surgery (rTOF) or were sham- operated (Sham). Following cardiac function assessment in vivo by MRI 3-4 months after surgery, pigs were euthanized and their hearts rapidly excised. Electrophysiological properties of right (RV) and left ventricles (LV) were obtained by optical mapping. Fibrosis was assessed histologically.
RV dysfunction was evident while LV function remained unaltered in rTOF pigs. LV action potential duration (APD) was significantly longer on the epicardium and endocardium of rTOF animals. RV epicardial and endocardial APD were not different between rTOF and Sham RVs. LV conduction velocity (CV) was significantly reduced in the longitudinal direction in rTOF pigs but remained unchanged in the transverse direction compared to Sham animals. In the RV both longitudinal and transverse CVs were significantly in rTOF compared to Sham. An elevated collagen content was found in both the LV and RV of rTOF pigs. A trend for a lateralization of Connexin43 was found in the LV of rTOFs which, together with the increased fibrosis, may account for the reduced longitudinal conduction velocity in this ventricle.
Altogether, these findings highlight the presence of a pro-arrhythmic substrate in the ventricles of rTOF pigs.
-
Jean-Frederic Gerbeau, PhD: INRIA, France
Jean-Frédéric Gerbeau, PhD: INRIA, France
Forward and inverse problems in computational electrocardiology
Jean-Frédéric Gerbeau1,2
1Inria Paris-Rocquencourt
2Université Pierre et Marie Curie
This talk will be devoted to an overview of results in the numerical simulation of the electrical activity of the heart.
First, the forward problem of electrocardiology, namely the direct simulation of electrocardiograms, will be addressed. It will be based on a biophysical model of the heart [1], including a surface model of the atria [2]. An application to the simulation of a magneto-hemodynamics artifact in MRI will be shown [3].
Then, the talk will address a few questions about the inverse problem of electrocardiology. In the literature, this question is usually tackled by regularizing an ill-posed inverse problem to reconstruct the epicardial potential. Instead, our approach consists of identifying the state and the parameters in our forward model. In this context, reduced order strategies prove to be useful [4]. To improve the identifiability of the model, a promising approach is to use multimodality measurements, including both electrophysiology and mechanics. Preliminary results based on synthetic data will be shown.
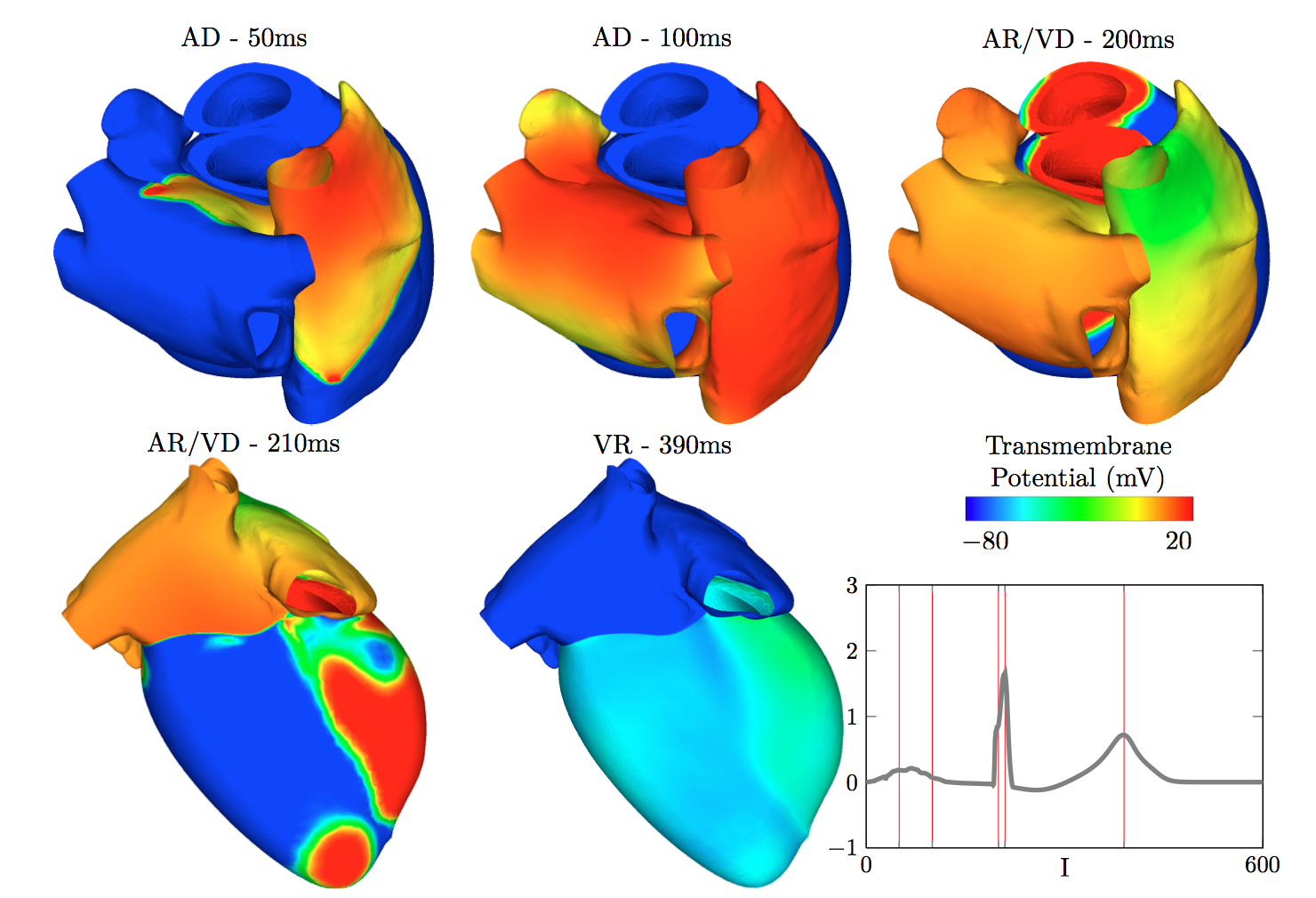
Action Potential in the heart and the corresponding ECG (A. Collin, J-F. Gerbeau, E. Schenone, in preparation)
References
[1] M. Boulakia, S. Cazeau, M. Fernandez, J.-F. Gerbeau, and N. Zemzemi, “Mathematical Modeling of Electrocardiograms: A Numerical Study,” Annals of Biomedical Engineering, vol. 38, no. 3, pp. 1071-1097, MAR 2010, 2010.
[2] D. Chapelle, A. Collin, and J.-F. Gerbeau, “A surface-based electrophysiology model relying on asymptotic analysis and motivated by cardiac atria modeling,” Mathematical Models & Methods in Applied Sciences, vol. 23, no. 14, pp. 2749-2776, DEC 2013, 2013.
[3] V. Martin, A. Drochon, O. Fokapu, and J.-F. Gerbeau, “MagnetoHemoDynamics in the aorta and electrocardiograms,” Physics in Medicine and Biology, vol. 57, no. 10, pp. 3177-3195, MAY 21 2012, 2012.
[4] M. Boulakia, E. Schenone, and J.-F. Gerbeau, “Reduced-order modeling for cardiac electrophysiology. Application to parameter identification,” International Journal For Numerical Methods in Biomedical Engineering, vol. 28, no. 6-7, pp. 727-744, JUN- JUL 2012, 2012.
Animal and In-Vitro Experiments
Session chair: Irene Vignon-Clementel, PhD: INRIA, France
-
Guiti Milani, MD: Necker-Enfants Malades Hospital, France
Guiti Milani, MD: Necker-Enfants Malades Hospital, France
Experimental work on right ventricular outflow tract : from clinical issus to animal work and back to clinical application
Tetralogy of Fallot (TOF) is one of the most frequent congenital heart defect. Patients are usually operated within the first months of life. Several type of surgical techniques are used to treat the right ventricular outflow tract depending on the initial anatomy : pulmonary valve commissuroplasty, transannular patch or interposition of a conduit between the right ventricle and the pulmonary arteries. Most patients with TOF reach adulthood but many of them will need reinterventions. The type of reintervention will depend of the initial treatment and type of residual lesions. For many years, pulmonary regurgitation, direct consequence of transannular patch technique was not a concern. However over the last decade, many armful consequences of free pulmonary regurgitation on the right ventricular future have been demonstrated.
Our team has always been directly involved in the care of patients with TOF and residual lesions. Patients with degraded RV-PA conduits were treated by cardiac interventions using bare metal stents to relief stenosis. By definition this treatment was leaving the patient with free pulmonary regurgitation.
Our team has done experimental work to implant a valved stent. The valved stent could relief stenosis but also treat pulmonary insufficiency. It was developped by our team : a bovine jugular valve was sutured in a bare metal and it was implanted in ewes. Acute experiments were conducted to prove technical feasability followed by chronic studies to demonstrate sustained valvar function. After promising results, the valved stents were implanted in patients with great succes.
Implantation of these valved stents is now one of the main succes stories of the last decade in congenital heart disease. But this technology is theoritically so far limited to patients with RV-PA conduits. This technique is currently limited to patients with a right ventricular outflow tract no bigger than 22 mm in diameter because of intrisic characteristics of the valve. However, TOF is mostly treated with transannular patch and in this setting, patients usually present with large outflow tracts.
Two reserach pathways were explored to expand the use of the valved stent to large outflow tracts : hybrid approach and new devices. Hybrid procedure have been developped in the animal laboratory: a combination of surgery and interventional techniques and feasability of this procedure has been demonstrated. New devices to create a platform for the implantation of the valved stent have been studied and developped : these devices are called infundibular reducer. Successful implantation have been published.
The next step is implantation of these infundibular reducer in patients but one of the limitions is the various shapes of the infundibulum. In the future, collaborative work with engineers, cardiac MRI and radiologists will help to design patient-specific device using reconstructed anatomies and computer simulations.
-
Jonathan Butcher, PhD: Cornell University, USA
Jonathan Butcher, PhD: Cornell University, USA
Distinct roles of RhoA and Rac1 during hemodynamic remodeling of embryonic heart valves
Russell A. Gould1 BS, Huseyin C. Yalcin PhD2, Joanna L. MacKay3 PhD, Sanjay Kumar4 MD, PhD, Jonathan Butcher1 PhD
1 Department of Biomedical Engineering, Cornell University, Ithaca, NY 14853, USA 2 Department of Mechanical Engineering Department, Dogus University, Istanbul 34722, TURKEY 3 Department of Chemical and Bimolecular Engineering, University of California Berkeley, CA 94720, USA 4 Department of Bioengineering, University of California Berkeley, CA 94720, USAEmbryonic valves rapidly evolve from disorganized globular “cushions” to highly organized thin fibrous leaflets. These multi-scale interactions occur within a demanding hemodynamic environment and are essential to maintain unidirectional flow. The mechanisms by which mechanical forces direct cellular response to coordinate this cycle of morphogenesis and function are not known. In this study we profile two mechanosensitive small GTPases, RhoA and Rac1, and establish how they coordinate atrioventricular valve (AV) differentiation and morphogenesis. RhoA activity is elevated during early cushion formation, but decreases considerably over development. In contrast, active Rac1 increases in cushions as they mature into valves. Using gain and loss of function assays, we determined that the RhoA-SRF pathway is essential for the myofibroblastic contractile phenotype of early cushion mesenchyme, but surprisingly is insufficient to drive matrix condensation during valve maturation. The Rac1-p38 pathway in contrast restricted myofibroblast differentiation, but was necessary and sufficient to drive cell adhesion, polarization, and enhanced stress fiber alignment essential for directed matrix condensation during valvular remodeling. RhoA drives Rac1 inactivation in mechanically stressed cells via FilGAP binding. Importantly, we establish that cyclic stretch uniquely inhibited RhoA activity and caused nuclear membrane localization of FilGAP, which in turn directly activated Rac1 signaling. Finally, we used partial atrial ligation experiments to confirm that altered hemodynamic loading in vivo augmented or restricted cushion growth directly through potentiation of RhoA and Rac1 activity. Together, these results establish that cyclic mechanical signaling is essential to remodel endocardial cushions into thin leaflets via coordinating RhoA to Rac1 based signaling.
-
Richard Figliola, PhD: Clemson University, USA
Richard Figliola, PhD: Clemson University, USA
In vitro Multi-domain Modeling of the Univentricular Circulation
Richard Figliola, PhD1
1 Clemson University, Clemson, SC 29634
The Fontan circulation is the common surgical pathway for patients born with only one fully functioning ventricle. This surgical correction uses a three-stage palliation strategy. After birth, an operation (Stage 1) is performed to improve the flow of oxygenated blood in the systemic circulation while retaining flow to the pulmonary circulation. Subsequent surgeries (Stages 2 and 3) reconstruct the circulation routing the total venous return directly to the pulmonary circulation.
Most engineering studies have focused on the hemodynamics of the surgical anastomosis site without coupling this to a patient’s circulation, thus limiting the scope of systems-level predictive studies, particularly under the time-dependent conditions. Newer multi-domain models couple accurate models of the surgical reconstruction with a lumped parameter model of the surrounding circulation tuned to clinically measured, patient-specific physiological impedances. Given the heterogeneous physiologies of this population and the desire for improved surgical modifications, such improved patient-specific in vitro models bring a meaningful translation tool to explore surgical strategies and devices.
This presentation will describe multi-domain experimental models of the univentricular circulation representative of each stage of the Fontan. Patient-specific impedances, based on clinical measurements, are shown to permit physiologically realistic time-based pressures and flows. Ventricular work is applied, as well as respiration in the Stage 3 model, to include consequential time-dependent effects. In particular, the models are used to show the systems level effects of recurrent coarctation, vascular resistance effects on flows to the different territories, as well as the important respiration impact on these circulations with metabolic activity.
-
John LaDisa, PhD: Marquette University, USA
John LaDisa, PhD: Marquette University, USA
Analysis of hemodynamic stimuli, persistent vascular changes and gene expression in a rabbit model of human aortic coarctation and repair
John F. LaDisa, Jr., Ph.D.1,3 and Thomas J. Eddinger, Ph.D.2
1 Department of Biomedical Engineering, Marquette University 2 Department of Biological Sciences, Marquette University 3 Herma Heart Center, Children’s Hospital of Wisconsin; Milwaukee, WisconsinCoarctation of the aorta (CoA) is a constriction of the descending thoracic aorta and is one of the most common congenital cardiovascular defects. Despite correction, patients often have a reduced life expectancy from increased cardiovascular morbidity, most notably hypertension, as well as early onset coronary artery disease and aneurysm formation. Identifying the causes of this morbidity is difficult given the heterogeneity (e.g. follow-up time, coarctation severity, concomitant anomalies) and limited number of CoA patients at a given center. We developed an animal model to control these variables and introduce CoA using a clinically-representative 20 mmHg pressure gradient. This model mimics aortic changes in humans, and facilitates study of corrected CoA by using dissolvable sutures to induce the coarctation. While the stimuli for aortic alterations and morbidity are reversed for ~6 human years after the suture dissolves in corrected rabbits, restoring pressure alone does not alleviate increases in medial thickness and stiffness, or decreases in contractility to phenylephrine and endothelial dysfunction denoted by acetylcholine vs sodium nitroprusside induced relaxation. Results also show a phenotypic shift in medial smooth muscle from contractile to synthetic for CoA and corrected rabbits. Microarray techniques were used to quantify differentially expressed genes (DEG) in the upstream aorta that experiences hypertension following surgical induction of CoA, and restoration of pressure after correction. These DEG offer additional insight into potential mechanisms of persistent morbidity in CoA. Translational studies are now underway to quantify the impact of coarctation severity, age of onset, and duration of stimuli on these DEG.
-
Vincent Fleury, PhD: Paris Diderot, France
Vincent Fleury, PhD: Paris Diderot, France
Direct in vivo imaging (time-lapse) of cell to organ morphogenetic coupling
Vincent Fleury1
1 Biofluidics Group, Laboratoire Matière et Systèmes Complexes, Université Paris Diderot/CNRS, 10 rue Alice Domont et Léonie Duquet 75013 Paris, FranceOrgan morphogenesis is a very dynamic process which involves considerable movement and reorganization, at the tissue scale. In recent years, physicists have started to study the many aspects of organ morphogenesis with classical tools of condensed matter physics, such as visco-elasticity measurements, conservation laws, symmetry principles etc. In our group, we focus on early stages of vertebrate development, and try to understand the transition from a flat discoid embryo, at the blastula stage, to a recognizable bilateral animal with rudiments of eyes, heart, vertebrae precursors, limb plates etc. This occurs quite rapidly in many animals such as fish, frog or chicken. Mammals are slower, though, and more difficult to image. However, we manage to film chicken development, at cell resolution scale, from the “as received” chicken embryo to a recognizable vertebrate, and measure the mechanical properties of different tissues. These movies allow one to understand the mechanism by which a 3D complex animal is formed, starting from a flat simple discoid tissue. Actually, there exist rings of cells inside the disc, having different elastic properties. The boundaries between these rings of cells serve 3 purposes : they separate cell-differentiated territories; they localize the lines along which mechanical forces are exerted; they lock the position of the fold furrows during formation of the embryo body. Grouping these 3 issues along the same boundaries ensures a robust and reproducible morphogenetic process, able to segregate different physiological functions in physically separated domains, as so often observed anatomically, and filmed here in vivo with high definition.
Tetralogy of Fallot
Session chair: Silvia Schievano, PhD: UCL, UK
-
Adam Dorfman, MD: University of Michigan, USA
Adam Dorfman, MD: University of Michigan, USA
Tetralogy of Fallot: an Opportunity for Collaboration
Tetralogy of Fallot is the most common cyanotic congenital heart lesion, occurring in approximately 30-40 of 100,000 live births. It is characterized by anterior malalignment of the conal septum, resulting in a ventricular septal defect (VSD) with aortic override, right ventricular outflow tract (RVOT) obstruction and right ventricular hypertrophy. Surgery is usually performed in the first six months of life, with patch closure of the VSD and relief of RVOT obstruction.
Residual lesions often persist or develop following repair. These most frequently include pulmonary regurgitation, scarring of the right ventricular outflow tract and right bundle branch block (RBBB) with prolongation of the QRS interval. Surgical management is not a true “repair”; these residual lesions lead to increasing morbidity and mortality as patients age.
These long-term problems represent an opportunity for collaboration between clinicians and engineers to address hemodynamic and electrophysiologic challenges; for example, the collaborative work using patient-specific cardiovascular magnetic resonance data coupled with computer-based fluid-structure interaction models to address individualizing the approach to RVOT reconstruction (Tang D et al, J Biomech Eng 2008).
There are many unsolved problems to address, often in determining ideal timing for pulmonary valve replacement. Collaboration with engineers may provide clinicians improved understanding of how to optimize the efficiency of ventricular work. Additionally, the interaction of the conduction system with ventricular function in patients with RBBB and the role of biventricular pacing could be explored. These collaborative data can hopefully be linked to improved outcomes, bringing fields together to improve the lives of our patients.
-
Alfonso Caiazzo, PhD: Weierstrass Institute, Germany
Alfonso Caiazzo, PhD: Weierstrass Institute, Germany
Efficient blood flow simulations for the design of stented valve size reducer in enlarged ventricular outflow tracts
Alfonso Caiazzo1, Romain Guibert2, Younes Boudjemline3, Irene E. Vignon-Clementel4
1 Weierstrass Institute for Applied Analysis and Stochastics (WIAS), Berlin, Germany 2 Institut de Mécanique des Fluides de Toulouse (IMFT) Toulouse, France 3 Service de Cardiologie Pédiatrique Hôpital Necker-Enfants Malades, Paris, France 4 INRIA Paris-Rocquencourt and UPMC Université Paris 6, FranceTetralogy of Fallot (TOF) is a congenital heart disease characterized over time, after the initial repair, by the absence of a functioning pulmonary valve, which causes regurgitation, and enlarged right ventricle outflow tract. Due to this pathological anatomy, available valve devices are usually too small to be deployed in the enlarged tracts. To avoid additional complex surgery in these patients that suffer from pulmonary insufficiency, an al- ternative consists in implanting these valves percutaneously in combination with a size reducer stent. We describe a computational model to study the effect of a stented valve size reducer on the hemodynamics in enlarged ventricular outflow tracts. To this aim, blood flow in the right ventricular outflow tracts is modeled via the incompressible Navier-Stokes equations, which are solved numerically using a finite element method. Numerical sim- ulations are based on a patient geometry obtained from medical imaging and boundary conditions tuned according to available measurements of inlet flow rates and pressures. In order to investigate multiple device configurations and to describe the effect of the geometry on hemodynamics, we construct different geometrical models of the reducer, varying its length and/or di- ameter, also investigating how to reduce computational complexity using a reduced order model based on Proper Orthogonal Decomposition (POD). We monitor the forces exerted on the valve and on the reducer depending on the geometrical parameters. Moreover, computational results support the thesis that the reducer does not introduce additional pressure gradients.
-
Frandics Chan, MD, PhD: Stanford University, USA
Frandics Chan, MD, PhD: Stanford University, USA
Tetralogy of Fallot: Contribution of MRI and CT to Clinical Practice, Treatment Planning, and Research
Tetralogy of Fallot (TOF) is the most common cyanotic heart disease, representing 10% of all cases of congenital heart disease. Today, total surgical repair at early childhood greatly enhances survival and quality of life of these patients. In most cases of simple TOF, the imaging needs for initial diagnosis and surgical planning are met by echocardiography. More complicated cases, where there are additional intracardiac anomalies, abnormal pulmonary artery anatomy, malformation of the airways and lungs, may require catheterization, CT, or MRI to supplement diagnostic information.
The most frequent and important clinical indication for cardiac MRI in patient with TOF is the evaluation of impending right heart failure after total surgical repair. In young patients, total repair of TOF calls for closure of the ventricular septal defect and relief of the subvalvular, valvular, supravalvular pulmonary stenosis, the latter often accomplished with transannular patch augmentation. This leaves a varying degree of pulmonary regurgitation. While most patients tolerate the additional volume load to the right ventricle, about 10% of these patients progress to right-heart failure, necessitating surgical or transvascular pulmonary valve replacement. Since all artificial valves have limited longevity and once placed they will likely require future replacement, this operation is ideally done just before irreversible RV failure. In current clinical practice, this event is estimated by ventricular sizes and ejection fractions. Cardiac MRI provides the most accurate measurements of these markers. The precise thresholds for these markers are being investigated by ongoing clinical studies.
Despite the demonstrated utility of cardiac MRI and the clinical needs to follow an increasing number of patients with repaired TOF, the availability of high-quality MRI study remains limited outside academic centers. The reasons may be traced to the high cost, the length, and complexity of the examination. These limitations are workflow related and may be ameliorated by volumetric acquisition of anatomic and flow information with 4D phase-contrast (4DPC) imaging technique. In the past few years, the performance of 4DPC in terms of acquisition time, temporal resolution, and image quality have improved significantly. Versions of 4DPC are being implemented by scanner manufacturers. User friendly, efficient software programs are becoming available for visualization and quantitative analysis of the 4DPC image data. Deployment of these technologies facilitates cardiac MRI study of patients with congenital heart disease, including TOF.
Besides clinical applications, cardiac MRI and CT are power tools for the investigation of pathophysiology and experimental treatments for TOF. Both MRI and CT provide detailed 3D or 4D structural information of the heart and the pulmonary vasculatures needed for computational fluid dynamic (CFD) experiments. In addition, MRI phase contrast imaging adds boundary information for CFD calculations. Finally, cardiac MRI has unique capabilities for myocardial tissue characterizations. These capabilities include myocardial tagging for strain mapping, T1-mapping and delay-enhancement for scar imaging, first-pass contrast enhancement for perfusion imaging, and diffusion imaging for myocardial fiber tracking. The information may assists construction of patient-specific or treatment-specific heart models to evaluate the effectiveness of treatments.
-
Maxime Sermesant, PhD: INRIA, France
Maxime Sermesant, PhD: INRIA, France
Reduced-Order Statistical Models of Cardiac Growth and Motion in Tetralogy of Fallot Patients
This work is focused on statistical modelling of cardiac growth and motion to enable patient-specific predictions from a population-based analysis. The first part aims at building a statistical growth model through aging. It was applied to Tetralogy of Fallot patients to model the complex evolution of the ventricles due to the pathology. A reduced representation of the shape evolution was proposed, as well as its correlation with different clinical indices. The second part concerns the development of a statistical model of cardiac motion at a population level. A reduced-order cardiac-specific motion model was proposed to represent the motion dynamics with a small number of parameters. From the computed transformations, the parameters were analysed using statistical methods to obtain population-based measures of normality. A mean motion model was derived to represent the normal motion for a given population. The presented methods allow to apply a statistical analysis on high dimensional objects (shapes, deformation fields), so that main characteristics of a patient group can be extracted. This can be used to better understand the pathology as well as stratify patients and plan therapy.
Day-2, May 22, 2014
Respiratory and Cardiovascular Modeling in Practice
Session chair: Miguel Fernandez, PhD: INRIA, France
-
Ira Katz, PhD: Air Liquide, France
Ira Katz, PhD: Air Liquide, France
An Analytical Formulation to Calculate Pressure Distributions of Gas Mixtures in an Infant Airway Morphology Model
Ira Katz1,2, Laure Gouinaud1, Georges Caillibotte1
1 R&D Medical Gases Group, Air Liquide Santé International, Les Loges-en-Josas, France 2 Department of Mechanical Engineering, Lafayette College, Easton PA, USAAn analytical model for pressure loss has been used to simulate the inhalation pressure distribution in a healthy nine-month old infant lung morphology model. The model was previously validated for adults. Pressure distributions are calculated for air as well as helium and xenon mixtures with oxygen to investigate the effects of gas density and viscosity variations for this age group. The results indicate that there are significant pressure losses in infant extrathoracic airways due to inertial effects leading to much higher pressures to drive nominal flows in the infant airway model than for an adult airway model. For example, the pressure drop through the nasopharynx model of the infant is much greater than that for the nasopharynx model of the adult; that is, for the adult-versus-child the pressure differences are 0.08 vs. 0.4, 0.16 vs.1.9, and 0.4 vs. 7.7 cm H2O, breathing helium-oxygen (78/22%), nitrogen-oxygen (78/22%), and xenon-oxygen (60/40%), respectively. Within the healthy lung viscous losses are of the same order for the three gas mixtures, so the differences in pressure distribution are relatively small.
-
Celine Grandmont, PhD: INRIA, France
Celine Grandmont, PhD: INRIA, France
Mathematical and numerical modelling of some aspects of the respiration
Celine Grandmont1,2
1Project-team REO, Inria Paris-Rocquencourt
2 LJLL laboratory, UMPC, France
What are the main mechanisms involved in an asthma crisis?
How does a curative aerosol or the dust pollution deposit in the airways?
How emphysema or fibrosis affects the supply of air?
From the medical, environmental and societal point of view, this kind of questions raises many challenging issues. In this talk we will review how a mathematical approach of these questions could provide new elements and improve the understanding on the topic. In particular, numerical simulations of well calibrate models may accurately reproduce the behaviour of the airflow or the aerosol deposition in the bronchial tree to further help the clinician to identify the pathology and cure it.
-
Agnes Pradel, MD: AP-HP Groupe hospitalier Pitié France
Agnes Pradel, MD: AP-HP Groupe hospitalier Pitié France
Unveiling the mechanics of healthy and pathological tracheobronchial trees during forced expiration
A. Pradel1, K. Blanc1, P. Gilfriche1, T. Similowski1, M. Filoche2 ,C. Straus1
1 Université Pierre et Marie Curie, UMR S 1158 2 Physique de la Matière Condensée, Ecole PolytechniqueFlow-volume loops measured during forced expiration are one of the gold standard measurements in Pulmonary Function Test (PFR). This maneuver imposes on the pulmonary airway system a mechanical challenge that allows one to detect possible alterations of the lung. The numerical simulation of this maneuver requires solving in a coupled way the fluid transport and the tube mechanics in about 30,000 bronchi. To that end, we use an asymmetric model of the tracheobronchial tree in which the mechanics is described by tube laws that relate the local diameter of a bronchus to the local pressure difference across the bronchus wall. The fluid transport equations are integrated along the main direction of each bronchus, leading to a lumped parameter model of the entire tree.
This model has been first validated on published flow-volume loops for various gas mixing (oxygen, helium, SF6...). Secondly, experimental measurements of forced and tidal flow-volume loops have been performed on healthy and pathological subjects. During the maneuvers, the pleural pressure has been recorded. On the same subjects, the tracheal diameter has been evaluated using an acoustical method. Using these measurements, we have been able to accurately reproduce the flow-volumes in healthy subjects, and detect pathological behavior.
Finally, several scenarios of pathological conditions have been tested. In particular, it has been observed that a significant obstruction of the distal bronchi might remain undetected through forced expiration maneuver whereas this technique exhibits a higher sensitivity to modifications in the proximal region.
This work has been supported by the ANR program SAMOVAR ANR-2010-BLAN-1119.
-
Ryo Torii, PhD: UCL, England
The Application of Computational Biomechanics in Clinical Science – Bicuspid Aortic Valve and Ross Procedure
Ryo Toriia, X. Yun Xub and Magdi H. Yacoubc,d
a Department of Mechanical Engineering, University College London, Torrington Place, London WC1E 7JE, UK.
b Department of Chemical Engineering, Imperial College London, Exhibition Road, London SW7 2AZ, UK
c Harefield Heart Science Centre, Harefield Hospital and Imperial College London, Hill End Road, Harefield UB9 6JH, UK
d Qatar Cardiovascular Research Center, Qatar Sci. and Tech. Park, Doha, Qatar
The aortic valve and root perform extremely sophisticated functions that are critically dependent on their topology as well as the structure of their component parts at tissue, cellular and molecular levels. Each of these components is capable of changing its size and shape during different phases of the cardiac cycle. Computational biomechanics offers unique capabilities to visualise, quantify and predict biomechanical functions under conditions that mimic the in vivo environment.
In this talk, the application of computational biomechanics to the study of the aortic root in health and disease as well as following different types of valve preserving and aortic root replacement using biological valves will be presented. Example applications include: (1) localised damage to the aortic aneurysm wall due to haemodynamic jet in bicuspid aortic valve patients and (2) distensibility analysis of the pulmonary autograft after the Ross procedure.
Although model assumptions are inevitable, careful problem setup according to specific study objectives could guide one to make appropriate use of mathematical modelling tools to address questions with clinical endpoints. Integration of the computational approach with medical imaging and experimental methods is a key to achieve this.
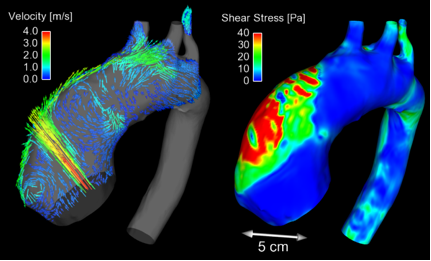
Fig 1. Flow patterns (left) and wall shear stress (right) in the aneurysmal aorta of a BAV patient (Torii, et al. JACC Cardiovasc Img. 2012; 6).
Single Ventricle Heart
Session chair: Francesco Migliavacca, PhD: Politecnico di Milano, Italy
-
Tain-Yen Hsia, MD: Great Ormond Street Hospital, England
Tain-Yen Hsia, MD: Great Ormond Street Hospital, England
Leducq Transatlantic Network of Excellence in Multi-Scale Modeling of Single Ventricle Hearts for Clinical Decision Support
Children born with only one ventricle are consigned to a lifetime of surgeries and abnormal physiology. While a three-stage operative strategy has reversed an otherwise uniformly fatal disease, the complex interplay of man-made anatomy, changing physiology with growth, and deranged fluid dynamics continues to plague the understanding and care of these patients throughout the world. Additionally, multiple operations exist for each stage, with unknown consequences. Advanced engineering and imaging methods hold much promise to improve the care of these patients, but investigations that solely solve the local dynamics of the operative reconstruction (micro- scale) without incorporating global, systemic influences (macro-scale) are not clinically useful. Single ventricle physiology clearly exhibits a highly variable multi-scale behavior that changes with patient-specific parameters, type and age of surgical repair, and pharmacological/clinical interventions.
Since 2010, a group consisting of 4 US and 3 European institutions have embarked on collaborative research program supported by a 5-year grant from Leducq Foundation (Paris, France) to integrate our expertise in pediatric cardiology, surgery, imaging, engineering, and computer science to develop a modeling system that can assist and support the clinical management of patients with single ventricle hearts. We set out 4 major aims:
Aim 1: Construct multi-scale models of all three Stages of HLHS physiology, and the surgical/hybrid alternatives for each, based on patient specific parameters obtained from clinical investigations.
Aim 2: Validate the multi-scale modeling to aid surgical planning, predict clinical hemodynamics, and guide patient management. Discrepancies between clinical parameters and modeling predictions will be used to modify and improve the mathematical models. Concurrent rigorous validation will also be conducted with in vitro experimental mock circuits of each palliative stage.
Aim 3: Characterize patient-specific contractility-afterload mismatch between the single ventricle and the surgically reconstructed aorta.
Aim 4: Develop an integrated software solution to implement the algorithms and techniques defined in Aims 1, 2, and 3 to allow world-wide access to this software product.
In this talk, we will present the progress achieved during the last 4 years that have assist clinical decision making, and to discuss future plans and yet uncovered questions that can benefit from innovative engineering solutions.
-
Giancarlo Pennati, PhD: Politecnico di Milano, Italy
Giancarlo Pennati, PhD: Politecnico di Milano, Italy
Central and modified Blalock-Taussig shunts: CFD patient-specific models*
Chemistry, Materials and Chemical Engineering ‘Giulio Natta’ Dept., Politecnico di Milano, Milan, Italy
Hemodynamics of single ventricle patients at the first stage palliation is very complex due to the presence of a conduit parallelizing the systemic and pulmonary circulations. Customized multi-domain models including central shunt and modified Blalock-Taussig shunt are presented to investigate local such as vortex flows in the conduit, retrograde flows in the upper body branches and coronary blood flow steal.
Three-dimensional models of the patients shunts included the aortic arch, the upper branches and the left and right pulmonary arteries. These models are coupled to the lumped parameter network reproducing the circulation, including the heart and the coronary circulation.
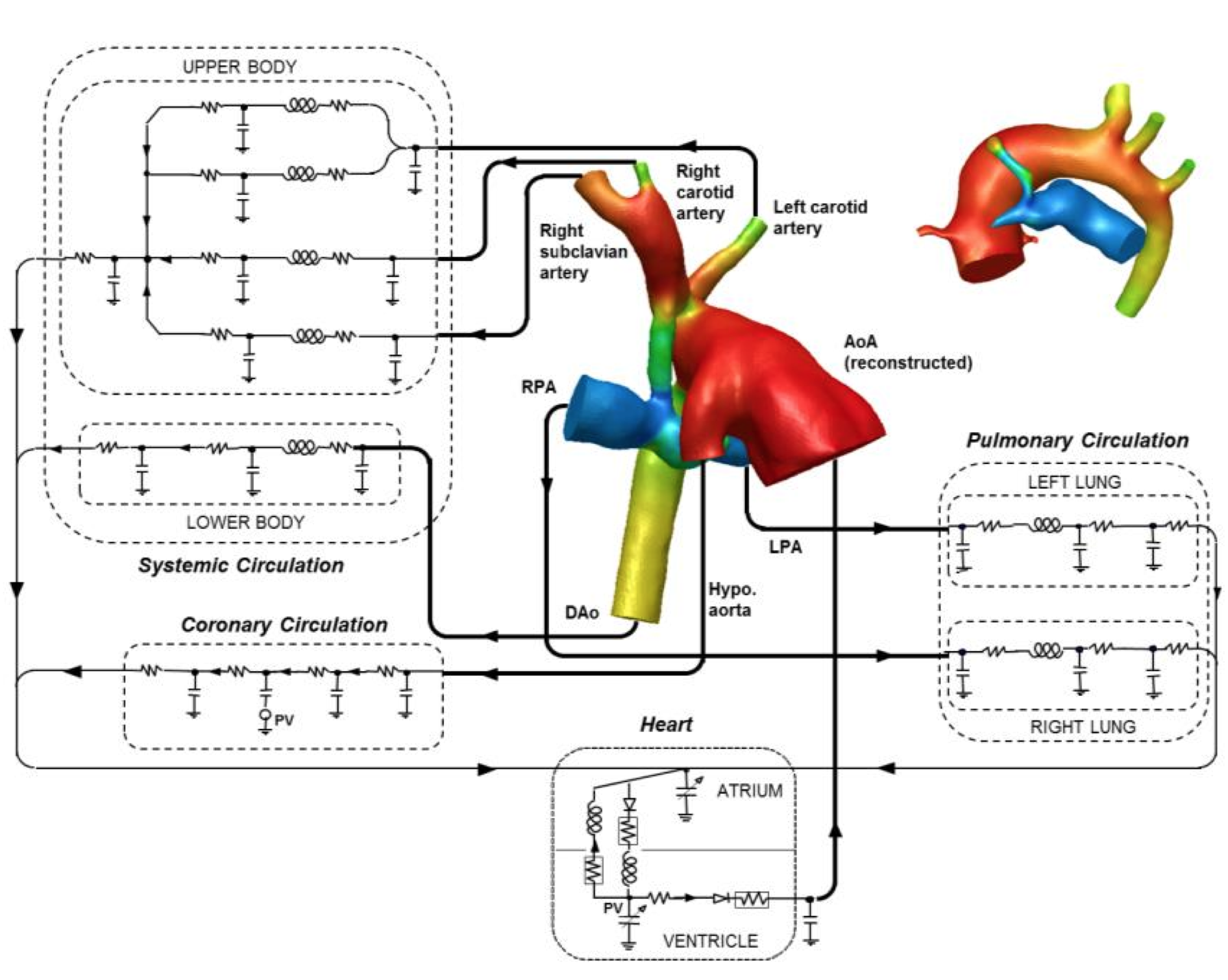
Results show that the multi-domain models are able to reproduce the hemodynamic quantities of clinical interest, i.e. aortic, pulmonary artery and atrial pressures, cardiac output and flow repartition between upper and lower body, left and right lungs. Reported errors are reasonable (< 20%), given the uncertainties related to the non-simultaneous acquisition of flow rates and pressures, the estimation of pulmonary arterial pressures as pulmonary venous wedge pressures, and the imaging resolution. These limitations will be discussed as they are unavoidable with such small patients and complex circulations.
*This work has been done within the framework of the Transatlantic network of Excellence ‘Multi-scale modeling of single ventricle hearts for clinical decision support’ of the Fondation Leducq with the collaboration of the Modeling Of Congenital Hearts Alliance (MOCHA) Group.
-
William DeCampli, MD: University of Central Florida, USA
William DeCampli, MD: University of Central Florida, USA
SINGLE VENTRICLE HEART DISEASE IN THE BME LABORATORY
William M. DeCampli 1 , M.D., Ph.D and Alain J. Kassab 1 , Ph.D
1 University of Central Florida Colleges of Medicine and Engineering and Computer Science, Orlando, FLBackground: As computational models (CFD) contribute new knowledge and clinical direction in single ventricle congenital heart disease, the need for complementary studies in the biomechanics and animal laboratories takes on increasing importance.
Methods: We describe three areas of laboratory investigation in progress at our program at UCF that complement our ongoing CFD efforts. These include (1) coronary blood flow in shunt physiology, and a proposed device to “counterpulsate” a shunt, (2) biomechanical properties of native vessels and graft materials encountered in congenital cardiac surgery, with application to fluid-structure interaction (FSI) in CFD and (3) bench top circulatory models.
Results: (1) An aortopulmonary shunt decreases coronary blood flow (CBF). Counterpulsation of the shunt graft restores coronary blood flow to pre-shunt values while maintaining substantial shunt-derived pulmonary blood flow. (2) Using neonatal piglets as a human analogue, we show that average “stiffness” of the great vessels depends on the vessel, axis (circumferential vs. longitudinal), location (proximal vs. distal) and orientation (ventral vs. dorsal). Differences may exceed a factor of 3-4. Realistic FSI in CFD must incorporate the anisotropic stress/strain relationships of native and patch material. (3) Using a bench top model of ventricular assist device (VAD) flow, we show that optimization of VAD outflow cannula implant orientation may reduce the risk of cerebral thromboembolism by 40-60%. CFD studies corroborate these results, which we are currently generalizing to pulsatile flow.
Conclusions: CFD and laboratories dedicated to physiological and biomechanical behavior complement each other in furthering our knowledge of single ventricle congenital heart disease.
-
Alison Marsden, PhD: University of California, San Diego, USA
Alison Marsden, PhD: University of California, San Diego, USA
Simulation of Assisted Bidirectional Glenn: Early Results and Future Directions
Mahdi Esmaily Moghadam1, Tain-Yen Hsia2, Alison Marsden1
1 MAE Department, UCSD, San Diego, CA 2 Cardiac unit, GOSH, London, UKFor single ventricle heart patients, palliation with systemic-to-pulmonary shunts (SPS) remains unsatisfac- tory. In a prior optimization study, we found that limited improvement can be achieved compared to standard shunt geometries if we limit ourselves to the SPS anatomy. Also, early palliation with the bidirectional Glenn (BGLN) anastomosis is discouraged because the neonatal superior vena cava (SVC) may be unable to pro- vide adequate pulmonary blood flow. Hence, we proposed a novel approach where additional flow in a Glenn would be ‘assisted’ by a shunt between the innominate artery and SVC. Motivated by the ejector pump concept in fluids mechanics, improved pulmonary blood flow can be achieved with only a modest increase in SVC pressure. In this study, a multiscale modeling framework, following prior numerical studies of single ventricle physiology, is adopted to examine the ABG and compare it to the SPS and BGLN. Two levels of pulmonary vascular resistance are simulated to model the neonatal and pre-stage two conditions. The results show that the ABG provides the highest oxygen saturation and oxygen delivery among all surgeries, leading to significantly lower heart load compared to the SPS and higher pulmonary blood flow compared to the BGLN. The SVC pressure, however, is also the highest in the ABG, ranging from 8-15 mmHg depending on the pulmonary vascular resistance. This study demonstrates that the ABG is a compelling alternative to current surgical methods that warrants further investigation with in vitro and in vivo models.
-
Hao Liu, PhD: Chiba University, Japan
Hao Liu, PhD: Chiba University, Japan
Patient-specific modeling–based assessment of cardiovascular function
H. Liu1,2, F. Liang2, K. Sughimoto3, K. Tsubota1
1 Graduate School of Engineering, Chiba University, Chiba, Japan 2 Shanghai Jiao Tong University and Chiba University International Cooperative Research Center, Shanghai Jiao Tong University, Shanghai, China 3 Department of Cardiac Surgery, The Royal Children's Hospital, VIC, AustraliaCardiovascular function assessment based on in silico patient-specific modeling might become a powerful tool in the surgical decision-making process. For this purpose, we have developed a multi-scale, multi-physical biomechanical simulator for the cardiovascular system (CVS), which consists of a closed-loop, lumped-parameter (0D) compartment model of the global CVS, a one-dimensional (1D) hemodynamic model, a systematic control model of the autonomic nervous system (ANS) with consideration of the cerebral auto-regulation (CA), and an image-based three-dimensional (3D) computational fluid dynamics (CFD) model of local arteries and organs. Here we present the framework of the biomechanical simulator and our recent results of its application in the assessment of cardiovascular function relating to cardiovascular surgery including: 1) patient-specific modeling of off-pump coronary artery bypass graft surgery and assessment of cardiovascular function by combining computational hemodynamics and clinical data; 2) computational modeling and prediction of transient hemodynamic changes upon changing a bidirectional cavopulmonary anastomosis (BCPA) into a total cavopulmonary connection (TCPC) in staged Fontan operation; 3) CFD-based theoretical analysis of the fenestration size effects on Fontan circulation and surgical process; and 4) computational modeling of thrombus formation associated with Fontan routes of TCPC and atrio-pulmonary connection (APC).
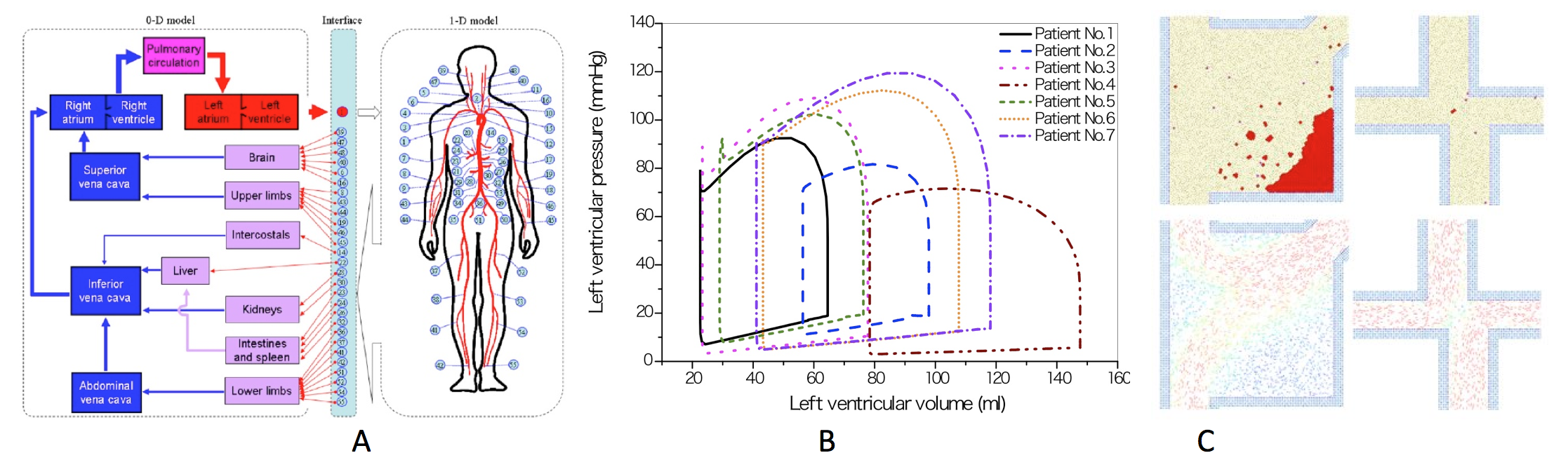
Figure 1. A. Schematic description of multi-scale modeling of the human cardiovascular system. B. Model-predicted left ventricular pressure–volume loops for seven patients. C. Thrombus formations in Fortan routes of TCPC & APC.
References
1 F. Liang et al. Transient hemodynamic changes upon changing a BCPA into a TCPC in staged Fontan operation: a computational model study, The Scientific World Journal. doi:10.1155/ 2013/486815, 2013.
2 K. Sughimoto et al. Assessment of cardiovascular function by combining clinical data with a computational model of the cardiovascular system, The Journal of Thoracic and Cardiovascular Surgery. 145(5): 1367-72, 2013.